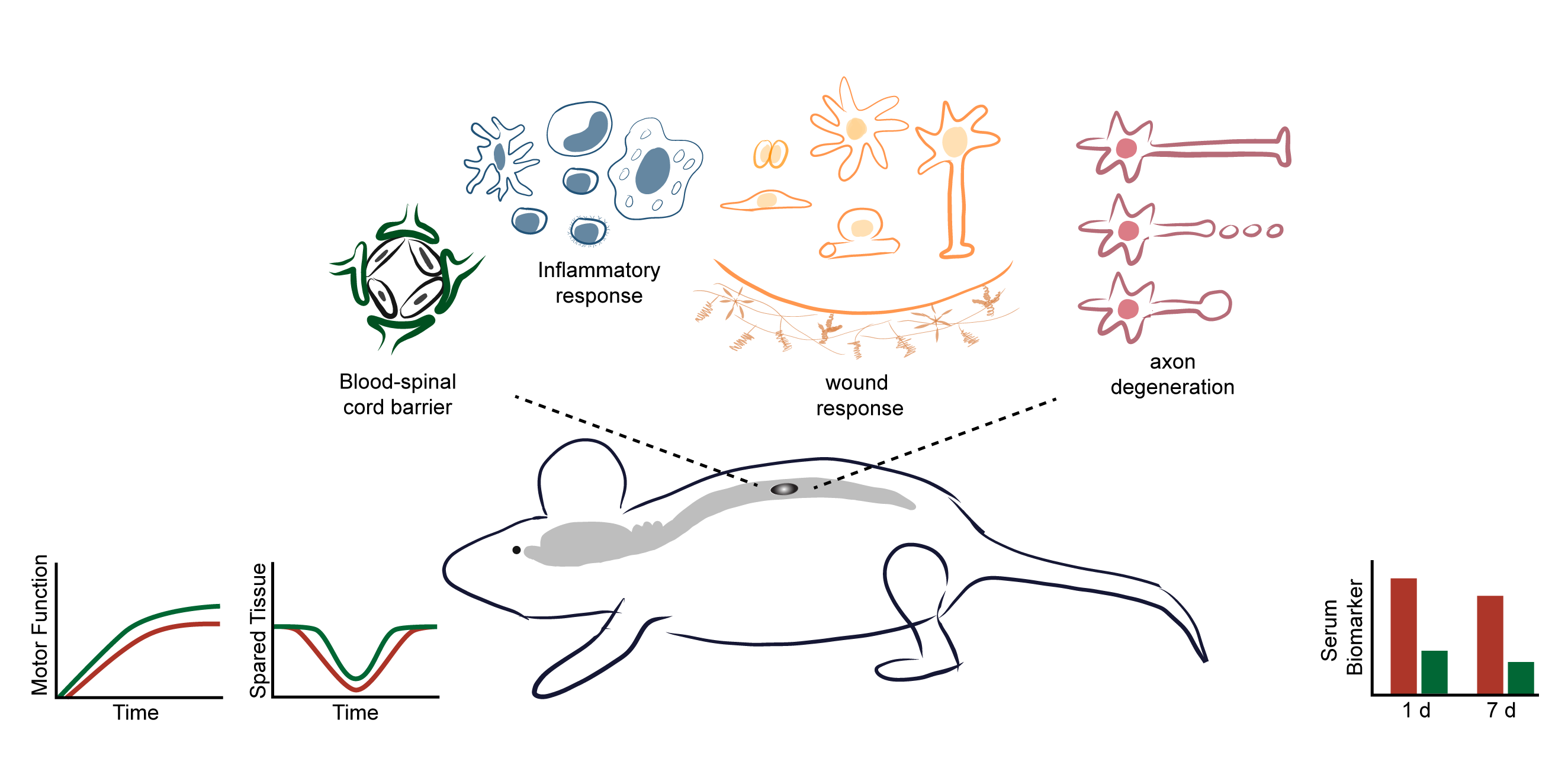
The Hill laboratory focuses on spinal cord injury (SCI) repair and the development of therapeutic interventions to promote recovery of function. Studies range from examining the basic biology of axonal regeneration to testing small molecules and cellular transplants for their ability to promote axonal regeneration and functional recovery in rodent models of spinal cord injury.
Spinal cord injury
Injury to the spinal cord causes direct damage to the cells within the spinal cord. This damage, referred to as the primary injury, results in the activation of biochemical cascades which results in damage to adjacent cells that were initially undamaged. This spread of damage is referred to as the secondary injury. Over the subsequent days to weeks, the injury site undergoes endogenous changes. The balance of the detrimental changes associated with the primary and secondary injury and the beneficial and detrimental changes associated with the endogenous wound healing response ultimately determines the amount of function retained.
Testing of therapeutic interventions
Therapies for SCI focus on different aspects of the cellular and biochemical changes that occur. Early interventions focus on minimizing the spread of tissue damage or altering the progression of the cellular changes in order to preserve as much tissue and function as possible. In the lab, we test promising pharmacological interventions to promote and restore function using preclinical SCI models. To assess the outcomes of treatment, we use in vivo imaging, behavioral testing (e.g., open field locomotion, ladderwalk, gait analysis, and thermal and pressure sensation), histology (e.g., cryosectioning, immunohistochemistry, epifluorescence and confocal microscopy), biochemistry (e.g., Western blotting), and molecular biology (e.g., quantitative PCR). Many of these same outcome measures are also used when we examine the temporal changes in cells that occur following SCI. For these studies, we are identifying additional cellular changes that occur following SCI in order to identify and develop new therapeutic interventions.
In collaboration with Karyopharm, a pharmaceutical company that develops selective inhibitors of nuclear export for various human diseases, we recently received a grant from the Department of Defense to test the ability of a specific nuclear export inhibitor to promote SCI repair. This grant seeks to generate the data necessary to move the compound forward through the pre-clinical to clinical pipeline. The studies to be performed will assess the cellular and molecular mechanisms of action and optimize the drug dosing schedule necessary for the submission of a pre-IND.
Use of cellular transplants
SCI results in the disconnection of circuitry necessary for motor and sensory function. Cellular transplants can assist in preserving tissue and/or restoring circuitry. Several cell types are currently in Phase 1 clinical trials for SCI repair and their use for human SCI therapies appears to be safe. A variety of different cell and tissue types have been transplanted into spinal cord injury models (e.g., peripheral nerves, Schwann cells, olfactory ensheathing glia, fetal tissue, neural stem/progenitor cells, induced pluripotent cells). The specific effects of a given cell type differ, but in general cell transplants can release growth-enhancing factors to preserve tissue, provide a substrate for axons to grow on, replace damaged neurons to establish network relays, and/or replace damaged glia to ensheath or myelinate axons to help restore axonal conduction. Alone, cell transplants are not enough to re-establish substantial function following SCI; however, they could be an essential component of a combination strategy for SCI repair.
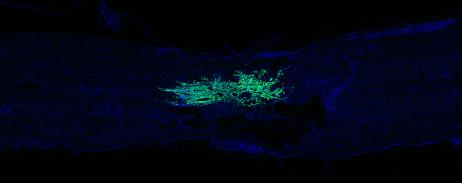
Over the past decade, we have worked on overcoming some of the limitations of cellular transplants in order to improve their efficacy and facilitate functional recovery. One major area of research focus has been targeting acute transplanted cell death. Using in vivo imaging (IVIS), we established that the decrease in cells occurs over the first two days following transplantation. Currently, we are testing interventions that target this acute, narrow time window as an approach to promote the survival of the transplanted cells. For these studies, changes in survival are detected using microscopy and non-biased stereology or by using biochemistry via Western blotting.
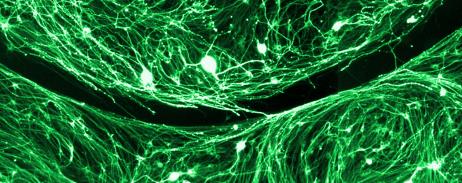
Another limitation of cell transplants is how they interact with residual spinal cord tissue. Using in vitro co-culture assays and in vivo spinal cord injury models, the interactions between cells following spinal cord injury are being examined. Currently, we are focusing on astrocyte interactions and how to improve the interaction of the cells at the lesion interface. Both the orientation and the integration of the cells at the edges of the transplant can negatively affect the ability of axons to grow into and out of the transplants. These experiments involve cloning, generation of viral vectors, in vitro co-culture assays, in vivo spinal cord injury modeling, immunohistochemistry, epifluorescence and confocal microscopy, axonal tract-tracing and behavioral testing.
Enhancing axonal growth
Following injury, most axons fail to regrow and connect to their targets. Instead, damaged axons form large, swollen endings that persist for months to years at the lesion margin. These altered endings may represent different types of endings formed in response to different inhibitory factors following injury or a common final phenotype of damaged axons. Surprisingly, little is known about the dystrophic endings that form following SCI. In the lab, we are working on identifying the subcellular changes that occur in axonal endings in vitro in response to inhibitory gradients. We have several lines of research in the lab to identify factors involved in dystrophic axonal ending formation and persistence in an effort to develop novel targets for chronically injured axons.
One of the challenges with targeting dystrophic axonal endings for SCI repair is the limited information currently available regarding the changes that occur within the endings. One of our goals is to establish a means to identify the pool of proteins that are altered within the endings in order to identify novel targets for SCI repair. These studies involve SCI modeling, tract-tracing, laser capture microdissection, RNA sequencing and mass spectrophotometry.
To identify potential therapeutic interventions, we are also currently screening pharmacological agents for their ability to increase axonal crossing of proteoglycan gradients using a modification of the spot assay originally developed by Dr. Jerry Silver’s lab. We are interested in further modifying this assay in order to establish a method for generating substrate-bound gradients that are compatible for use with microfluidics. This will facilitate the production of more endings for analysis. In addition to establishing methods to enhance the spot assay, we are using immunohistochemistry to identify and screen changes in major subcellular constituents to detect which subcellular factors differ between growth cones and dystrophic axonal endings. Together, these projects involve in vitro experiments to generate dorsal root ganglion neuron spot assay cultures, axonal tract-tracing and SCI modeling, and the assessment of axonal endings using immunohistochemistry and epifluorescence and confocal microscopy.
Our mission
The Hill lab is dedicated to the development and preclinical testing of strategies to enhance regeneration, repair and recovery of function following spinal cord injury. It is our mission that the laboratory be known for doing high quality, reproducible spinal cord injury studies with accuracy and integrity in an innovative and collaborative environment.
Publications
https://www.ncbi.nlm.nih.gov/myncbi/caitlin.hill.1/bibliography/public/
Current Positions of Past Trainees
Post-doctoral Fellows
- Ana Vivinetto, 2019 – Post-doctoral fellow, Laboratory for Neuronal Specification, Burke Neurological Institute, White Plains, NY. Mentor: Dr. John Cave.
- Paola Bianchimano, 2019 – Post-doctoral fellow, Visual Plasticity and Repair Laboratory, Burke Neurological Institute, White Plains, NY. Mentor: Dr. Botir Sagdullaev.
- Carolin Ruven, 2019 – Post-doctoral fellow, Laboratory for Cell Fate Specification and Circuit Development, Burke Neurological Institute, White Plains, NY. Mentor: Dr. Vibhu Saini.
- Ying Dai, 2017 – China
- Brian David, 2017 – Assistant Professor, Department of Neurosurgery, Rush Medical College, Chicago, IL. His lab focuses on SCI repair.
- Guiqian Chen, 2015 – Associate Professor, Department of Life Science and Medicine, Zhejiang Sci-Tech University. Hangzhou, China. His lab studies gene regulatory networks in craniofacial development using genetic mouse models.
- Sharnaz Kemal, 2014 – Post-doctoral fellow, Northwestern University, Chicago, IL.
- Veena Kandaswami, 2014 – Research Scientist, Lilly Research Laboratories, Eli Lilly and Company, NY, NY.
- David Coutts, 2012 – Senior Research Grants Manager, MS Society, London, UK.
Post-baccalaureate Fellows and Research Associates
- Jessica Curtin, 2019 – Research coordinator, Geneva Foundation, Keller Army Community Hospital / United States Military Academy, West Point, NY.
- Taylor Johns, 2018 – Technical associate, Sur lab, MIT, Cambridge, MA.
- David Goldberg, 2017 – Research Technician, Laboratory for Neuronal Specification and the Laboratory for Axonal and RNA Biology, Burke Neurological Institute, White Plains, NY. Mentors: Dr. John Cave and Dr. Diana Willis.
- Jennifer Brown, 2016 – Graduate Student working on both PhD and JD degrees. Graduate Program in Neuroscience at the University of Minnesota – Twin Cities, MN. Mentor: Dr.Sylvain Lesne.
- Kerri Scorpio, PhD, 2014 – Licensed Clinical Psychologist, Columbia Memorial Health, Hudson, NY.
- Chad Kurylo, PhD, 2012 – MBA Student, Cornell University, Class of 2020, NY, NY.
- Sarah Cook, DO, 2012 – Doctor of Osteopathy.
- Danika Brodak, MD, 2011 – Medical Fellow in Emergency Medicine, El Centro Regional Medical Center, affiliated with UC San Diego Health Care Network, El Centro, CA.
- Scott Raffa, MD, MBA, 2008 – Neurosurgeon and Spine Surgeon, Memorial Healthcare System. Hollywood, FL.
Mentored Student Presentations
- Emma Burns Optimizing cryoculture technique to generate dystrophic endings and growth cones for analysis. Burke Summer Scholars Program Poster Session. Aug 2018.
- Amanda Bache Comparative analysis of Zeb2os and ZEB2 in brain and spinal cord astrocytes. Burke Summer Scholars Program Poster Session. Aug 2018.
- Polin Petkova Analysis of the cellular components of dystrophic axonal endings in vitro. Burke Summer Scholars Program Poster Session. Aug 2016.
- Tanner Love Axonal regeneration failure: Investigating the role of protein synthesis and degradation in dystrophic axon endings. Burke Summer Scholars Program Poster Session. Aug 2015.
- Loren Eustaquio Testing in vitro assays to quantify viable Schwann cells. Burke Summer Scholars Program Poster Session. Aug 2014.
- Dylan Weil Comparison of protein degradation components in growth cones and retraction bulbs. High school project for Siemen’s’ Math Science and Technology competition. Jan 2014.
- Danika Brodak Assessment of proliferation of adult Schwann cells following transplantation into the injured spinal cord. Undergraduate Honors thesis as partial fulfillment of the requirements for magna cum laude degree at the University of Miami, May 2009.
- Scott Raffa The calpain inhibitor MDL28170 increases the survival of Schwann cells after transplantation into the injured adult rat spinal cord. A research thesis as partial fulfillment of the requirements for a Master of Arts in Medical Sciences degree from Boston University, Sept 2007.
- Inhibition of necrosis with the calpain inhibitor MDL28170 enhances the survival of Schwann cells transplanted into the injured adult rat spinal cord. Caitlin E. Hill, Scott. J. Raffa, Andres Hurtado, Mary Bartlett Bunge. 2nd place at the Florida Medical Association’s Annual Meeting / 3rd Annual Scientific Poster Symposium, Aug 2008.
- Yelena Guller In vitro models for enhancing adult Schwann cell survival: induction of necrosis and apoptosis and testing cell death-inhibiting drugs. Undergraduate Honors thesis as partial fulfillment of the requirements for magna cum laude degree at the University of Miami, May 2007.